Abstract
ZBTB7A is a transcription factor involved in the regulation of metabolism and hematopoietic linage fate decisions. Recently, we found ZBTB7A mutated in 23% of Acute Myeloid Leukemia (AML) patients with t(8;21) translocation (Hartmann et al., 2016, Nat Commun). However, the oncogenic collaboration between ZBTB7A alterations and the RUNX1/RUNX1T1 fusion in AML t(8;21) remains poorly understood.
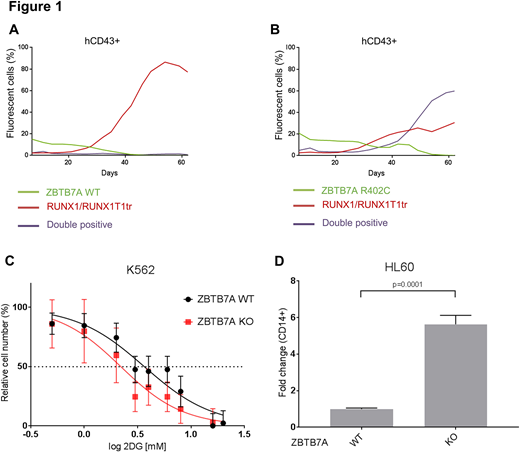
To study ZBTB7A mutations in the context of RUNX1/RUNX1T1-dependent transformation, we used human CD34+ cells co-transduced with a truncated form of RUNX1/RUNX1T1 and ZBTB7A wild-type (WT) or its mutants (R402C and A175fs). We then followed the evolution of fluorescence marker positive cells over a period of 60 days. While expression of RUNX1/RUNX1T1 alone caused clonal expansion, co-expression of ZBTB7A WT impaired the outgrowth of CD34+ cells (Figure 1a). In contrast, the anti-proliferative effect of ZBTB7A was lost for both of its mutants tested resulting in a rescue of the clonal expansion (Figure 1b).
To investigate the effect of ZBTB7A mutations on tumor metabolism, we used CRISPR/Cas9 to knockout (KO) ZBTB7A in the myeloid K562 cell line. As ZBTB7A is a known negative regulator of glycolysis, we treated KO and control cells with the glycolysis inhibitor 2-deoxy-d-glucouse (2-DG). KO cells were more sensitive to 2-DG compared to control cells (mean IC50: 3.06 vs 6.82 mM; p-value=0.087) (Figure 1c). These results are in line with the observed upregulation of glycolytic genes in ZBTB7A-mutant AML t(8;21) and suggest that these patients may benefit from the treatment with metabolic inhibitors. To learn more about deregulation of ZBTB7A target genes we are currently performing RNA-Seq analysis of WT vs KO K562.
Moreover, we used the K562 KO model to investigate the impact of loss of ZBTB7A on myeloid differentiation. The baseline expression of the erythroid marker CD235a was reduced in KO cells. Ectopic expression of ZBTB7A WT in the KO cells restored the CD235a levels to a control level, while expression of mutants or vector showed no effect. These findings are in agreement with previous reports of ZBTB7A involvement in erythroid differentiation.
To study the effect of ZBTB7A mutations on granulopoiesis, we established HL60 cells stably expressing WT or mutant ZBTB7A. We then differentiated the cells into granulocytes through all-trans retinoic acid (ATRA) treatment. Expression of WT, but not the mutants, resulted in a 4-fold increase of the granulocytic marker CD11b. Additionally, we induced monocytic differentiation through Phorbol 12-myristate 13-acetate (PMA) treatment. The mutant expressing cells showed similar levels of the monocytic marker CD14 as control cells. WT overexpressing cells had a 50% decrease in the number of monocytes. We then used CRISPR/Cas9 to establish ZBTB7A KO HL60, which exhibited a 5.5-fold increase in CD14 compared to control cells (Figure 1d). This data supports a previously unknown negative regulatory role of ZBTB7A in monocytic differentiation.
With regards to potential therapeutic applications, we tested the PMA sensitivity of HL60 and found a significantly lower IC50 in absence of ZBTB7A (mean: 124.5 vs 269.8 pM; p-value=0.001). Hence, loss of ZBTB7A may facilitate the pharmacological differentiation of leukemia cells.
In conclusion, ZBTB7A mutations in AML t(8;21) display a loss-of-function phenotype. Inactivating mutations of ZBTB7A allow for hCD34+ RUNX1/RUNX1T1-mediated expansion and deregulated tumor metabolism. Finally, loss of ZBTB7A expression perturbs myeloid development and thus may complement the block of differentiation induced by RUNX1/RUNX1T1. These findings open up avenues to novel therapies for ZBTB7A mutated patients including metabolic inhibition and pharmacological differentiation.
Hiddemann:F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal